Short answer: Rubber is made by (1) obtaining an elastomer (either natural latex from trees or synthetic polymers from monomers), then (2) compounding it with curatives, fillers, and stabilizers, (3) shaping it by extrusion or molding, (4) vulcanizing (curing) to create crosslinks, and finally (5) testing to standards before shipment.
The Two Origins: Natural vs. Synthetic
- Natural rubber (NR): harvested as latex from Hevea brasiliensis, then preserved, coagulated, and processed into sheets or TSR (technically specified rubber) blocks; latex may also be concentrated for dipping/foam.
- Synthetic rubber: produced by polymerization of petrochemical monomers (e.g., butadiene, styrene, acrylonitrile, isoprene) into elastomers such as SBR, NBR, EPDM, tailored for oil, temperature, or weather resistance.
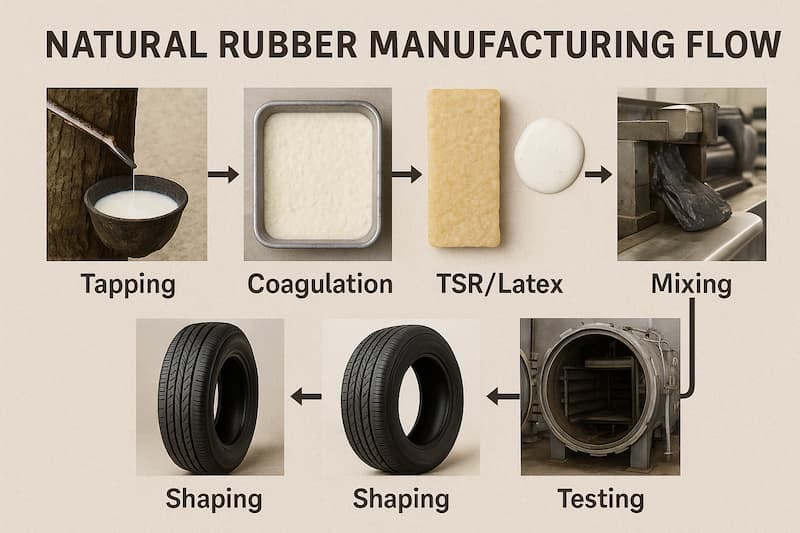
Natural Rubber Processing (Harvest → Sheet/TSR or Latex)
- Tapping & preservation: Trees are tapped; raw latex is filtered and preserved (e.g., with ammonia) to prevent premature coagulation for latex concentrates or sent to coagulate for dry rubber.
- Coagulation & milling: Latex is coagulated (e.g., with formic acid), rolled into sheets (RSS/ADS) or granulated, dried, and pressed into TSR blocks. TSR/SMR grades are lab-graded by dirt, ash, nitrogen, volatile matter (VM) and performance indices like Wallace plasticity (Po) and PRI; some supply specifications include Mooney viscosity bands and color.
- Why this matters: Cleanliness (low dirt/ash/N/VM) and stability (PRI/Mooney) drive mixing consistency, scorch behavior, and final properties—critical for tire and technical goods. (Ask vendors for a recent COA listing these metrics.)
Synthetic Rubber Polymerization (Monomers → Elastomer)
Refiners derive naphtha and related streams to produce monomers like butadiene and styrene; industry processes then polymerize these into elastomers (e.g., SBR, NBR, EPDM). Downstream processing pellets/bales the polymer for compounders. While the chemistry varies, all synthetic routes deliver an uncured polymer ready for compounding and cure.
Compounding: Where Properties Are Engineered
Compounding blends the base elastomer with ingredients that determine processing and performance:
- Curatives: Sulfur (classic vulcanization) or peroxides (for high-temp/chemical resistance). The industrial practice of vulcanization dates to the 1840s (Charles Goodyear’s patent in 1844).
- Accelerators & activators: Organic accelerators with zinc oxide/stearic acid activators form a zinc-sulfurating system that speeds crosslinking and controls network structure.
- Reinforcing fillers: Carbon black (abrasion, modulus) and silica (with coupling agents) tune strength, hysteresis, and rolling resistance. (Choice affects heat build-up and wear.)
- Plasticizers/process oils & resins: Adjust viscosity, low-temp flexibility, tack.
- Antioxidants/antiozonants & wax: Protect against oxygen/ozone cracking during service.
Mixing equipment: Industrial compounding typically uses Banbury-type internal mixers followed by mills/calenders; this is standard practice described in ASTM D3182.
Shaping and Vulcanization
- Shaping: After mixing, compounds are extruded (profiles, hose), calendered (sheet, fabric-rubber), or molded (compression, transfer, injection). (Process selection sets dimensional control and cure cycle.)
- Vulcanization (curing): Heat and pressure activate the cure system to form crosslinks that transform a sticky compound into an elastic, durable material—improving tensile strength, set, and heat/chemical resistance. Historically, heating rubber with sulfur is the classic method.
- Process history: The modern industry grew from Goodyear’s 1844 patent for vulcanized rubber.
How Finished Rubber Is Tested (Standards to Know)
- Mixing & specimen prep: ASTM D3182 covers materials/equipment/procedures for mixing standard compounds and preparing vulcanized sheets—useful to compare formulations consistently.
- Tensile properties: ASTM D412 measures tensile strength, elongation, and modulus of vulcanized rubber and TPEs.
- Hardness: ASTM D2240 (durometer) and ISO 48-4 (IRHD/Shore) define indentation hardness methods; 70 Shore A is a common general-purpose hardness for seals.
Table 1 — Ingredients & Their Roles (summary)
| Ingredient group | Typical examples | Primary purpose |
|---|---|---|
| Curatives | Sulfur, peroxides | Build crosslinks; set heat/chemical profile. |
| Accelerators/activators | TBBS, CBS; ZnO, stearic acid | Control cure rate/structure; activate sulfur. |
| Fillers (reinforcing) | Carbon black, silica + silane | Strength, abrasion, hysteresis control. |
| Process oils/resins | Naphthenic/paraffinic oils, tack resins | Processability, low-temp behavior. |
| Antidegradants | Aminic/phenolic antioxidants, PPD antiozonants | Resist oxidation/ozone cracking. |
Caption: Functional groups shown; exact chemistries vary by polymer family and service environment.
Table 2 — Use-Cases with Pros/Cons
| Process route | Typical products | Advantages | Watch-outs |
|---|---|---|---|
| NR latex → concentrate/dipping | Gloves, thread, foam, adhesives | High green-strength films; efficient dipping | Preservation/protein management; microbial control. |
| NR TSR/SMR → compounding | Tires, belts, mounts | Strong fatigue/abrasion; bulk supply | Batch variability: manage Mooney/PRI windows. |
| Synthetic (SBR/NBR/EPDM) → compounding | Hose, seals, wire/cable | Tailored resistance (oil, weather, steam) | Cure/package must match media & temperature. |
FAQ
- What are the main steps to make rubber? Obtain polymer (natural or synthetic), compound with chemicals, shape (extrude/mold), vulcanize, then test.
- How is natural rubber processed from trees? Latex is preserved, coagulated, rolled/dried into sheets or graded as TSR blocks; latex can also be concentrated for dipping.
- How is synthetic rubber made? Monomers (e.g., butadiene, styrene, acrylonitrile) are polymerized into elastomers then compounded and cured.
- What is vulcanization? A heat-activated chemical process (often sulfur) that creates crosslinks, improving strength and elasticity; widely adopted after 1844.
- Why add zinc oxide and stearic acid? They activate sulfur cures and, with accelerators, control crosslink formation.
- How are properties measured? Tensile by ASTM D412; hardness by ASTM D2240 / ISO 48-4; mixing & sheet prep per ASTM D3182.
- What equipment mixes rubber? Banbury/internal mixers and mills/calenders, then extrusion or molding.
- What goes on a rubber COA? Base polymer/grade and key test results (e.g., D412 tensile, D2240 hardness; for NR TSR: dirt/ash/N/VM, Po/PRI, Mooney).


