Rubber manufacturing is a multi-stage process that transforms raw latex or synthetic polymers into durable, engineered materials. From tires and seals to medical tubing, the way rubber is made directly affects its performance, safety, and sustainability.
Stage 1: Harvesting & Raw Material Preparation
Natural rubber begins with tapping Hevea brasiliensis trees. Latex is collected and preserved with ammonia to prevent premature coagulation.
Synthetic rubbers are produced through polymerization of monomers such as butadiene, styrene, or isoprene in large chemical reactors.
👉 Quality at this stage determines final product properties; impurities can weaken performance.
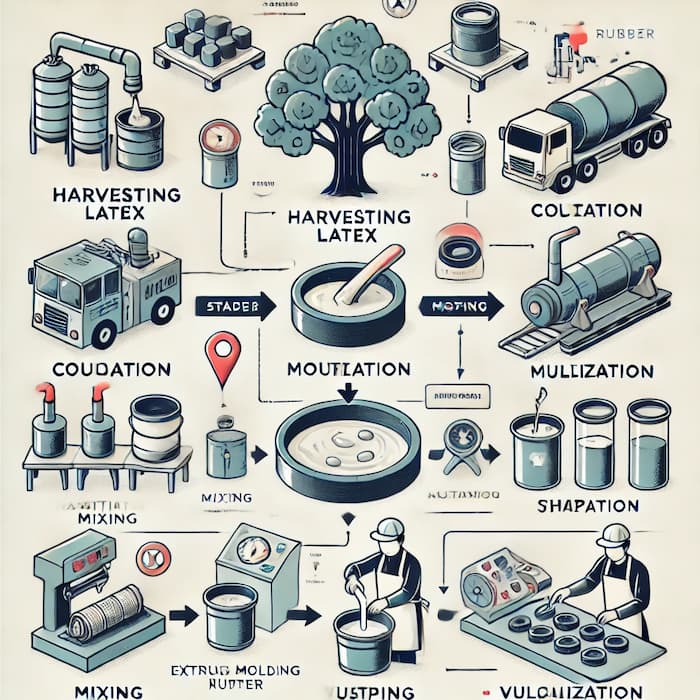
Stage 2: Coagulation, Drying & Mastication
- Coagulation: Latex is treated with acids (formic or acetic) to solidify.
- Drying: Sheets or TSR (Technically Specified Rubber) are air-dried or smoke-dried.
- Mastication: Rubber is mechanically worked in mills to soften it, making it easier to mix with additives.
Stage 3: Compounding & Mixing
Compounding is the art and science of rubber technology.
Ingredients include:
- Fillers (carbon black, silica) for strength.
- Plasticizers for flexibility.
- Antioxidants & antiozonants for durability.
- Accelerators & curing agents for efficient vulcanization.
Mixing occurs in Banbury mixers or two-roll mills; uniform dispersion is essential for consistent quality.
Stage 4: Shaping & Forming
The compounded rubber is shaped by:
- Extrusion: pipes, hoses, profiles.
- Molding: compression, transfer, injection molding for seals, gaskets, and precision parts.
- Calendaring: producing sheets, films, and coated fabrics.

Stage 5: Vulcanization (Curing)
Discovered by Charles Goodyear in 1839, vulcanization crosslinks rubber molecules with sulfur, turning sticky raw rubber into elastic, durable material.
- Parameters: temperature (140–180 °C), pressure, and time must be controlled.
- Modern curing agents include peroxides and specialty systems for high-performance rubbers.
Stage 6: Testing & Quality Control
Every batch undergoes rigorous tests:
- Mechanical: tensile strength (ASTM D412), hardness (ASTM D2240), compression set.
- Environmental: ozone resistance, heat aging, chemical resistance.
- Compliance: FDA, REACH, ISO 9001 standards ensure safety and reliability.
Sustainability & Innovations
- Recycling: devulcanization allows reuse of cured rubber.
- Bio-based rubbers: reduce dependence on petroleum feedstocks.
- Energy-efficient curing: microwave or UHF heating reduces cycle times.
Applications & End-Products
From tires to seals, hoses, medical devices, and infrastructure, the manufacturing process tailors rubber for each need.
The balance of compounding, shaping, curing, and testing ensures performance in real-world conditions.
Procurement Checklist
✅ Verify raw material origin and quality.
✅ Request compounding formula transparency.
✅ Ensure process control during curing.
✅ Review supplier QA/QC certifications.
✅ Confirm compliance with regulatory standards.
Conclusion
The rubber manufacturing process is more than a sequence of steps. It’s a controlled science that balances chemistry, mechanics, and sustainability. Understanding each stage helps engineers, procurement professionals, and students appreciate why rubber products perform the way they do—and how future innovations are shaping the industry.
FAQ
Q: What is the difference between natural and synthetic rubber manufacturing?
Natural rubber is harvested and coagulated, while synthetic rubber is polymerized from petrochemicals. Both follow compounding, shaping, and curing.
Q: Why is vulcanization important?
It transforms rubber from soft and sticky to durable and elastic by crosslinking polymer chains.
Q: Can vulcanized rubber be recycled?
Yes, through grinding and devulcanization, though processes are still evolving.
Q: What tests ensure rubber quality?
Tensile strength, hardness, aging resistance, and regulatory compliance checks are standard.


